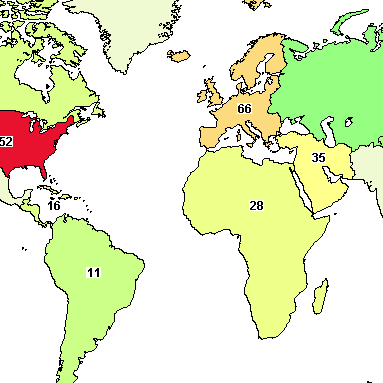
- Find out what Clinical Trials for Sickle Cell are Taking Place Around the World!A searchable map offered by ClinicalTrials.gov
- The Importance of Diversity Among Blood Donors: Unique Blood TypesPatients with Sickle Cell Disease are routinely transfused. It is [...]
- The Child with Sickle Cell Disease: A Teaching ManualWritten and Developed by Debra A. Vedro MSN RN CPNP [...]
- The Truth About TransfusionsEvery year more than 4 million American kids and adults [...]
- Sickle Cell News January 2021To join or leave the listserv visit https://scinfo.org/newsletter/ MARAC Advisory [...]
- Sickle Cell News for December 2020To join or leave the listserv visit https://scinfo.org/newsletter/ Help Spread [...]
- Sickle Cell News for November 2020To join or leave the listserv visit https://scinfo.org/newsletter/ SCDC UpdateOctober [...]
- Sickle Cell News for October 2020To join or leave the listserv visit https://scinfo.org/newsletter/ Spread the [...]
 Sickle Cell News from Around the Web
Sickle Cell News from Around the WebCity Spotlights
Learn about sickle cell resources in: Atlanta, Georgia; Oakland, California